Team : MULLE_CARTA
Research Projects
Synaptic dysfunction in relation to Alzheimer’s disease (AD)
Project Leader(s): Christophe Mulle - Thierry Amédée
AD is a progressive and devastating neurodegenerative disease, but despite considerable efforts in both fundamental and clinical research, it remain uncurable. AD is characterized by the extracellular deposit of Aβ plaques and the intraneuronal accumulation of hyperphosphorylated Tau. Our project address synaptic dysfunction in AD, which correlate well with early cognitive deficits, with a clear focus on presynaptic mechanisms (Barthet et al, 2018; Barthet and Mulle, 2020).
The amyloid precursor protein (APP) interacts with proteins of the presynaptic release machinery but the functional relevance thereof is poorly understood. We have shown that γ-secretase regulates presynaptic plasticity by controlling the proteolysis of APP (Barthet et al, 2018). We now address the pathophysiological function of presynaptic APP in controlling the activity of hippocampal circuits, and in particular inhibition/excitation balance.
Synaptic alterations are related, at least in part, to prominent neuroinflammation which contributes to the pathophysiology of AD. We have shown that a PGE2-EP3 signaling pathway is responsible for impairment of presynaptic LTP at Mf- CA3 synapses in APP/PS1 mice (Maingret et al., 2017). We now aim at deciphering the impact of neuroinflammation and activated microglia on synaptic function in the DG, in a mouse model of AD (APP/PS1 mice). We characterize synaptic function and morphology in the vicinity of amyloid plaques, in relation with microglial cells, and in conditions of pharmacological and/or genetic (opto- and chemogenetic) activation or inactivation of microglia.
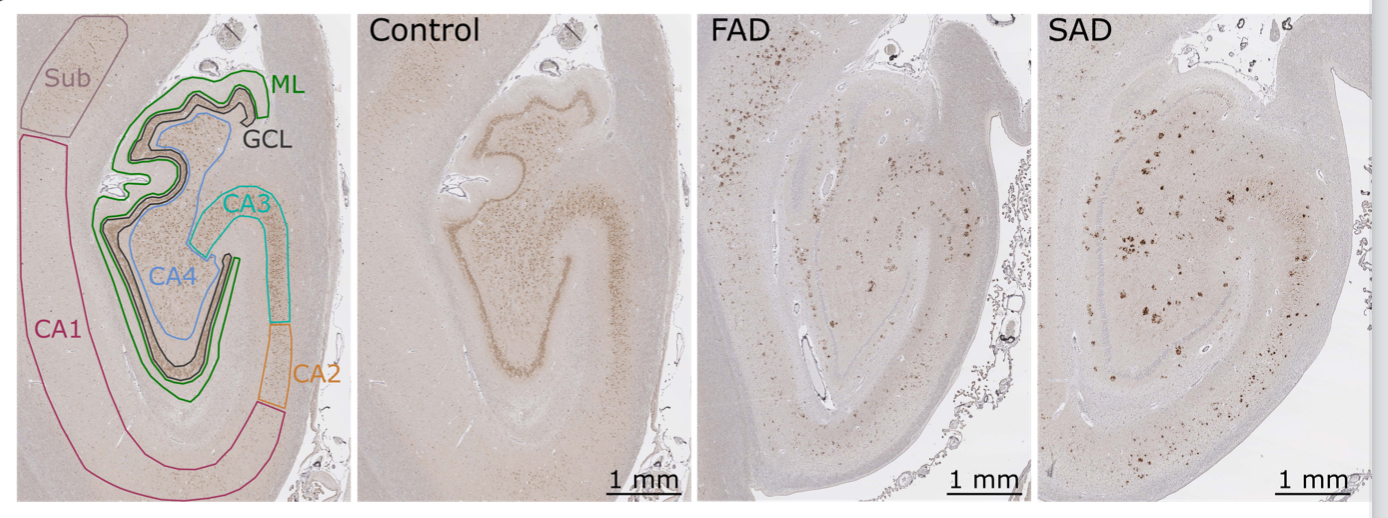
We are also interested in neuropathological features of APP in the human AD hippocampus. We combine an extensive set of methodological and analytical tools to investigate the presynaptic accumulation of APP around amyloid plaques in brain samples from patients with AD. This leads us to address defective trafficking of full length APP in the context of AD using mouse models of the disease.
APP accumulations in the hippocampus of AD patients. Human hippocampal sections are stained with an APP-Cter antibody. In both FAD and SAD cases, intense extra-somatic stainings are observable
Fundings
to fill